Is Crispr the Next Antibiotic?
In nature, the gene-editing tool Crispr protects bacteria against viruses. Now it's being harnessed in the fight against superbugs and the flu.

Send us a link
In nature, the gene-editing tool Crispr protects bacteria against viruses. Now it's being harnessed in the fight against superbugs and the flu.

The science of medicine is based on male bodies, but researchers are beginning to realize how vastly the symptoms of disease differ between the sexes - and how much danger women are in.

A fungus has been genetically modified with spider venom to kill the mosquitoes that spread malaria.

Misleading news claims can be detrimental to public health. We aimed to improve the alignment between causal claims and evidence, without losing news interest (counter to assumptions that news is not interested in communicating caution). We tested two interventions in press releases, which are the main sources for science and health news: (a) aligning the headlines and main causal claims with the underlying evidence (strong for experimental, cautious for correlational) and (b) inserting explicit statements/caveats about inferring causality. The 'participants' were press releases on health-related topics (N = 312; control = 89, claim alignment = 64, causality statement = 79, both = 80) from nine press offices (journals, universities, funders). Outcomes were news content (headlines, causal claims, caveats) in English-language international and national media (newspapers, websites, broadcast; N = 2257), news uptake (% press releases gaining news coverage) and feasibility (% press releases implementing cautious statements). News headlines showed better alignment to evidence when press releases were aligned (intention-to-treat analysis (ITT) 56% vs 52%, OR = 1.2 to 1.9; as-treated analysis (AT) 60% vs 32%, OR = 1.3 to 4.4). News claims also followed press releases, significant only for AT (ITT 62% vs 60%, OR = 0.7 to 1.6; AT, 67% vs 39%, OR = 1.4 to 5.7). The same was true for causality statements/caveats (ITT 15% vs 10%, OR = 0.9 to 2.6; AT 20% vs 0%, OR 16 to 156). There was no evidence of lost news uptake for press releases with aligned headlines and claims (ITT 55% vs 55%, OR = 0.7 to 1.3, AT 58% vs 60%, OR = 0.7 to 1.7), or causality statements/caveats (ITT 53% vs 56%, OR = 0.8 to 1.0, AT 66% vs 52%, OR = 1.3 to 2.7). Feasibility was demonstrated by a spontaneous increase in cautious headlines, claims and caveats in press releases compared to the pre-trial period (OR = 1.01 to 2.6, 1.3 to 3.4, 1.1 to 26, respectively). News claims-even headlines-can become better aligned with evidence. Cautious claims and explicit caveats about correlational findings may penetrate into news without harming news interest. Findings from AT analysis are correlational and may not imply cause, although here the linking mechanism between press releases and news is known. ITT analysis was insensitive due to spontaneous adoption of interventions across conditions. ISRCTN10492618 (20 August 2015)

Decades of early research on the genetics of depression were built on nonexistent foundations. How did that happen?

The Chan Zuckerberg Initiative will soon invite applications for open source software projects that are essential to biomedical research. Applicants can request funding between $50k and $250k for one year.

Superbugs are spreading. We need doctors trained to treat them.

The proportion of open-access publications with authors from the pharmaceutical industry doubled between 2009 and 2016.

Open Pharma, which works with pharma to drive fast and transparent medical publishing, is encouraging pharmaceutical companies to use their influence more.

The field has plenty of talented women, but to reach leadership roles they must have visible and recognizable roles within medicine and in the public

Academic research publications rely on doctors to voluntarily disclose their payments from drug and health companies in a lax reporting system some say is broken.

Misleading terminology and arbitrary divisions stymie drug trials and can give false hope about the potential of tailoring drugs to individuals, warns Stephen Senn.

Though there have been substantial efforts in increasing the racial and ethnic diversity in medicine, there has been minimal growth in people who identify as American Indian and Alaska Native and apply and enroll in medical school in the U.S., according to a new report released Tuesday.

Skeptics say drugs based on genetic insights have underdelivered. But look carefully and they're everywhere.
The sudden, unexplained removal of a research paper on private equity firms buying dermatology practices has raised questions about corporate influence.

Most scientists are satisfied in their jobs, but a significant number still face discrimination - an unacceptable situation.

A new wave of chatbots are replacing physicians and providing frontline medical advice-but are they as good as the real thing?
Drug companies make big contributions to analysis in the trials they fund but can fail to report their contributions.
Physician Peter Gotzsche, a board member of the organization, has been an outspoken critic of certain vaccines and of the pharma industry in general.

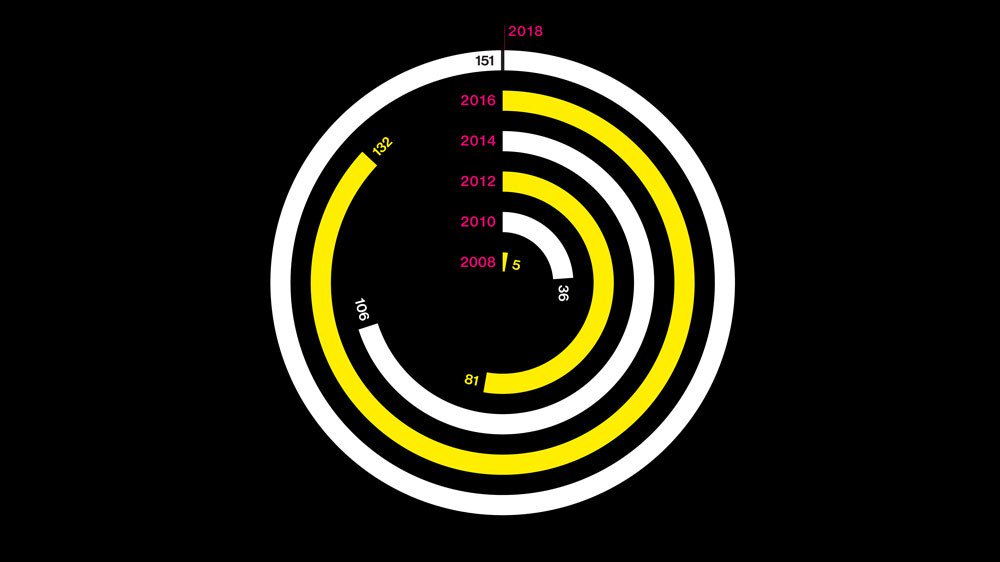
EU rules say clinical trial results must be reported onto the EU register for every trial there within 12 months of the end of the trial, yet no one has ever been sanctioned for breaking the European rules. European academic institutes are lagging far behind companies in complying with the reporting rules.

Study finds that compliance with the European Commission requirement for all trials to post results on to the EUCTR within 12 months of completion has been poor, with half of all trials non-compliant.
European Medicines Agency ends pharma evaluations work and moves contracts to bloc.

When conducting research involving the testing of human biospecimens, investigators and their institutions should routinely consider whether and how to return individual research results on a study-specific basis through an informed decision-making process, says a new report from the National Academies of Sciences, Engineering, and Medicine.
Many biologists are still reluctant to submit preprints, in part out of concern that doing so will allow others to “scoop” their work and undermine their chances of publication in a prestigious journal. I would like to rebut that concern, among others, and to share our research group’s first experience submitting a preprint manuscript.

An analysis of drug studies shows that most participants are white, even though trials are being done in more countries.
Life Science Alliance is a global, open-access, editorially independent, and peer-reviewed journal launched by an alliance of EMBO Press, Rockefeller University Press, and Cold Spring Harbor Laboratory Press.
It seems that CRISPR technology may indeed be all that it promises.
The breadth of social and moral questions raised requires a new architecture for democratic debate, insists Simon Burall.
If clinicians are expected to change their practice based on their reading of medical journals, they need to know that the evidence in published papers can be verified.
