Britain's Covid Research Led the World - Why Have Our Clinical Trials Fallen off a Cliff?
Britain's Covid Research Led the World - Why Have Our Clinical Trials Fallen off a Cliff?

Send us a link

NPP is committed to consistent and thorough reporting of clinical research which is essential for rigor, reproducibility, transparency, interpretation, and generalizability of published results to the broader human population.
Hundreds of trials have been disrupted in the medical research hub. Some patients are at risk of losing their last chance at survival.
Russia's invasion has the potential to disrupt clinical trials in Ukraine, warns one of the many companies staging trials in the nation.

How can we make sure that medical trials reported in the scientific literature are real? It is surprisingly hard - but not impossible.

Medical researchers are beginning to shift their focus away from COVID-19 - but the pandemic could continue to affect studies focused on other diseases.
Effort found four drugs had little benefit, now on hold until new drugs are chosen to test.
Expert behind vaccine confidence report points to halting of Oxford Covid trial as example.

With help from Fox News and Elon Musk, a misleading French study prompted a wave of misinformation that made its way to the president

Science investigation of ClinicalTrials.gov reveals that federal promises to enforce trial transparency have been ineffective.

Study investigates whether industry involvement in biomedical research affects trial design. A reduced use of active controls (such as alternate treatment or standard care) was found in trials with industry involvement, which can have the side effect of making results look more favourable than they actually are.
Assessment of risk of bias is regarded as an essential component of a systematic review on the effects of an intervention. The most commonly used tool for randomised trials is the Cochrane risk-of-bias tool. We updated the tool to respond to developments in understanding how bias arises in randomised trials, and to address user feedback on and limitations of the original tool.
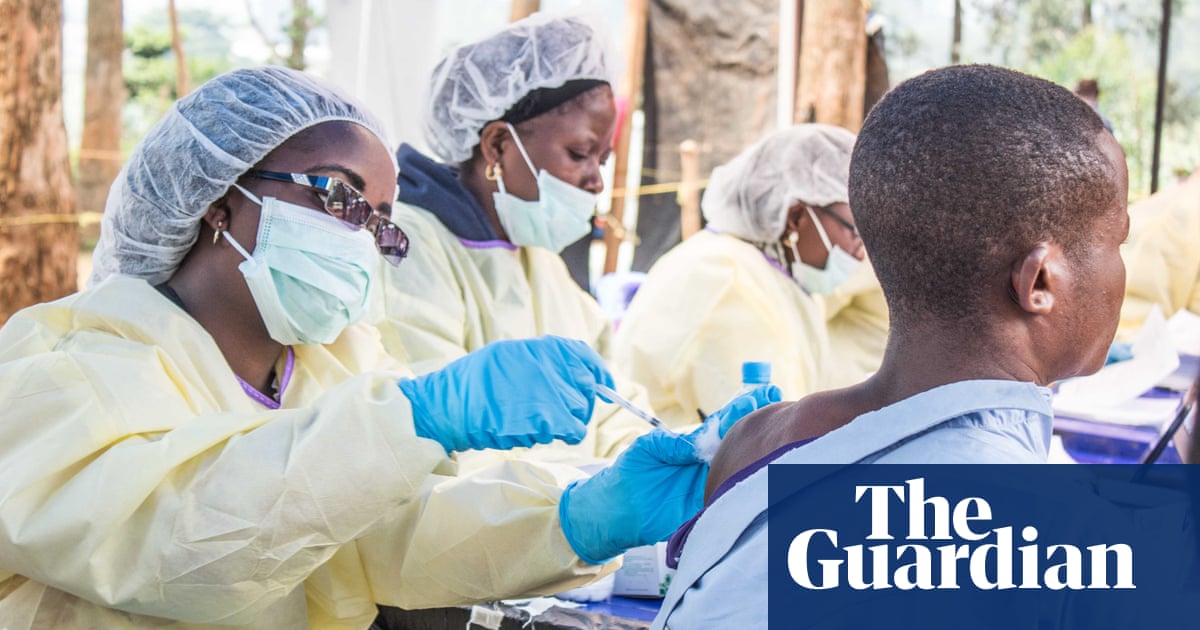
Experimental Ebola treatments carried out in the Democratic Republic of the Congo (DRC) have shown strong signs of being able to save patients’ lives.

Congo results show good survival rates for patients treated quickly with antibodies.
The failure to report the issue has not put patients at risk, the F.D.A. said, but the drugmaker could face criminal and civil penalties.
Analysis of 30 leading institutions found that just 17% of study results had been posted online as required by EU rules.

US law requires researchers to post study findings on a public registry within a year of completion - or face heavy fines.

The technologies that will be regulated by the ethics committee are often new and are deemed risky either because of safety or moral concerns.

UK universities could be brought in front of the House of Commons Science and Technology Committee if they fail to improve the reporting of clinical trials.
